Big news in the world of science and health – the FDA has given the green light to a groundbreaking treatment for sickle cell anemia using something called CRISPR. Let’s break it down in simple terms!
Understanding Sickle Cell Anemia
First off, what’s sickle cell anemia? It’s a health disorder where the red blood cells aren’t shaped like they should be. Instead of being round and flexible, they become sticky and form a crescent or “sickle” shape. The primary problem is that a protein (hemoglobin) found in red blood cells delivers oxygen to the body’s tissues. These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs). The recurrence of these events or crises can lead to life-threatening disabilities and/or early death. It is a condition that impacts the Black community most, but other communities can develop this condition as well.
What’s CRISPR, Anyway?
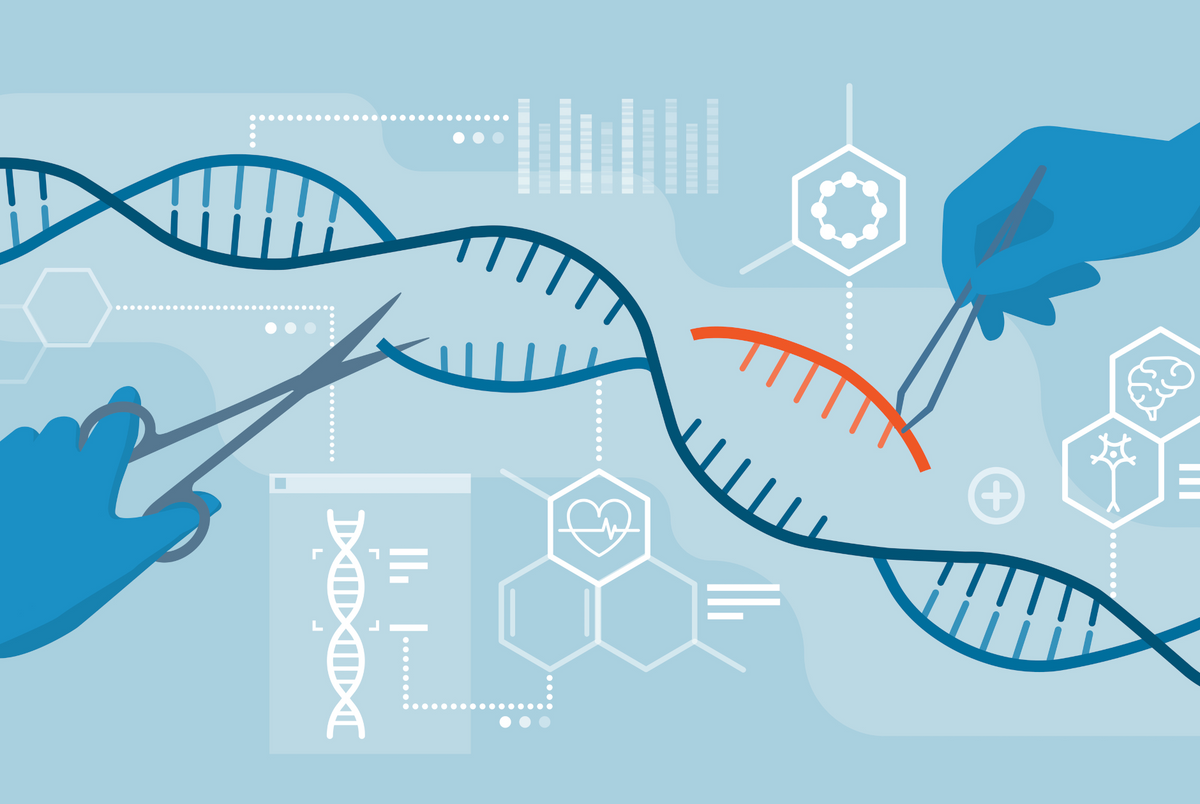
CRISPR might sound like a weird acronym, but it’s a super cool tool in the world of genetics. Imagine having a tiny pair of molecular scissors that can cut and edit your DNA – that’s CRISPR. Scientists use it to fix or change the genes causing problems in diseases like sickle cell anemia.
The Exciting FDA Approval
Now, let’s get to the exciting part – the FDA giving a thumbs up to gene therapy using CRISPR treatment for sickle cell anemia. The therapies are called Casgevy and Lyfgenia. This means scientists can use CRISPR to tweak the genes and help those with sickle cell live healthier lives. It’s like fixing a little typo in the genetic code to make everything run smoothly. Okay, so here’s the lowdown on how CRISPR works its magic. Scientists use it to target the specific gene causing sickle cell anemia (called HBB). With the molecular scissors, they snip out the problematic part and replace it with a healthy one. It’s like giving the cells a tiny makeover to work better!
What This Means for Individuals with Sickle Cell Anemia
For people dealing with sickle cell anemia, this FDA approval is a game-changer. The CRISPR treatment can potentially reduce the number of sickle-shaped cells in their blood, meaning less pain, fewer infections, and resolution of additional symptoms. It’s like opening up a new chapter of hope for individuals affected by this challenging condition.While this treatment shows enormous promise, it’s essential to mention the possible side effects. Some might experience mild reactions, like headaches or a bit of nausea. Scientists are always keeping an eye on these things to make sure the treatment is as safe as possible.
Wrapping It Up
In a nutshell, the FDA approval of Casgevy and Lyfgenia for sickle cell anemia is a big win for the sickle cell community, science, and health. It’s opening a door to a brighter future for those living with sickle cell anemia. As we cheer for this milestone, let’s stay excited about the possibilities ahead – more breakthroughs, more treatments, and more victories in the world of genetics!
The team at Chicago Genetic Consultants welcomes any questions about sickle cell anemia, so please contact us with any questions!